Views: 0 Author: Site Editor Publish Time: 2025-08-08 Origin: Site









Lithium tri-tert-butoxyaluminum hydride stands out as a strong candidate for selective reductions in organic chemistry. This reagent fills the gap between very strong, nonselective agents and milder, more selective options. Chemists often consider factors such as effectiveness, selectivity, safety, cost, and practicality when choosing the right tool. Lithium Tri-tert-butoxyaluminum Hydride: A Versatile Reducing Agent in Organic Chemistry offers a unique balance for many laboratory needs.
Chemists often look for reducing agents that can complete reductions quickly and completely. Effectiveness measures how well a reagent transforms a starting material into the desired product. Some reagents work best with certain types of chemical bonds. For example, lithium aluminum hydride can reduce many different functional groups, while others like sodium borohydride show more limited activity. The right choice depends on the substrate and the specific transformation needed. In many cases, a more effective reagent can save time and resources in the laboratory. Researchers also consider how much reagent is required for a complete reaction. Using less material can lower costs and reduce waste.
Selectivity describes a reagent’s ability to target one functional group while leaving others unchanged. This property is especially important when a molecule contains several reactive sites. Lithium tri-tert-butoxyaluminum hydride (LTBA) stands out for its chemoselectivity. Scientists have found that LTBA, sometimes used with sodium borohydride, can reduce aldehydes without affecting ketones, esters, amides, or nitriles. This makes LTBA a valuable tool for selective reductions in complex molecules. Other t-butoxy aluminum reagents, such as potassium diisobutyl-t-butoxyaluminum hydride, also show high selectivity. These reagents can reduce aldehydes and ketones to alcohols even when other sensitive groups are present. However, some reactions with LTBA may need longer times or higher temperatures. Selectivity helps chemists avoid unwanted side reactions and improves the purity of the final product.
Tip: High selectivity in reductions can simplify purification steps and increase overall yield.
Safety remains a top concern when choosing reducing agents. Some reagents react violently with water or air, which can create hazards in the lab. For example, lithium aluminum hydride can ignite if it contacts moisture. LTBA is less reactive with water, but it still requires careful handling. Chemists must use gloves, goggles, and work in a well-ventilated area or fume hood. Proper storage is also important to prevent accidents. Safer reagents can make laboratory work more efficient and reduce the risk of injury. Always review safety data sheets before starting any reductions.
Cost plays a major role when chemists select reducing agents for laboratory work. Some reagents come with a high price tag because of their complex synthesis or limited availability. Others, like sodium borohydride, offer a more budget-friendly option. Laboratories often need to balance performance with expense. When a project requires large-scale reductions, the cost of reagents can quickly add up.
Researchers must also consider the amount of reagent needed for each reaction. Some reagents work efficiently in small amounts, while others require excess to drive the reaction to completion. Using less material can help save money and reduce waste. Bulk purchasing sometimes lowers the price per unit, but not all labs have the storage space or budget for large orders.
Specialty reagents, such as lithium tri-tert-butoxyaluminum hydride, may cost more than common alternatives. However, their selectivity can justify the expense by improving yields and reducing the need for extra purification steps. In some cases, the higher cost of a selective reagent pays off by saving time and resources during workup and isolation.
Note: Always compare the total cost, including waste disposal and safety equipment, not just the price of the reagent itself.
Practicality measures how easy it is to use a reducing agent in real laboratory settings. Some reagents require special equipment or strict conditions, which can slow down routine work. For example, lithium aluminum hydride needs anhydrous conditions and careful handling. This requirement can make it less practical for beginners or for labs without advanced facilities.
Other reagents, like sodium borohydride, dissolve in water or alcohol and work at room temperature. These features make them more practical for many reductions. Chemists often choose reagents that fit their available tools and skills. A practical reagent should be easy to measure, mix, and handle safely.
Storage and shelf life also affect practicality. Some reagents degrade quickly or react with air and moisture. Labs must store these chemicals in special containers or under inert gas. If a reagent has a short shelf life, it may not be practical for infrequent use.
Tip: Choose reagents that match your lab’s equipment and experience level to avoid delays and accidents.
Practicality also includes how easily a reagent can be disposed of after use. Some reductions create hazardous waste that needs special treatment. Labs should plan for safe and legal disposal before starting any reaction.
Lithium tri-tert-butoxyaluminum hydride: a versatile reducing agent in organic chemistry, has a unique structure. The molecule contains an aluminum atom bonded to three bulky tert-butoxy groups and one hydride. This arrangement creates significant steric hindrance around the aluminum center. The large tert-butoxy groups shield the hydride, making it less reactive than in other metal alkoxyaluminium hydrides. This structure allows chemists to control the reactivity and selectivity of the reagent. The compound appears as a white to off-white powder and dissolves in common organic solvents. It remains stable when stored under inert gas and away from moisture.
The mechanism for reduction by lithium tri-tert-butoxyaluminum hydride: a versatile reducing agent in organic chemistry, relies on the addition of hydride to the carbonyl group. The bulky tert-butoxy groups slow down the reaction, which helps prevent over-reduction. Chemists often use this reagent to reduce esters to aldehydes without forming alcohols. The mechanism for reduction of esters follows these steps:
The hydride ion attacks the carbonyl carbon of the ester.
The alkoxy group leaves, forming an intermediate.
The bulky tert-butoxy groups on aluminum reduce the chance of a second hydride attack, so over-reduction does not occur.
During workup, the intermediate is protonated, yielding the aldehyde.
This stepwise process shows how steric hindrance from the tert-butoxy groups controls the reaction. Chemists often perform these chemical reactions at low temperatures, such as -78 °C, to increase selectivity and isolate the desired product. The mechanism for reduction is similar to that of other metal alkoxyaluminium hydrides, such as DIBAL-H, where steric effects also play a key role.
Note: The bulky tert-butoxy groups make lithium tri-tert-butoxyaluminum hydride: a versatile reducing agent in organic chemistry, especially useful for selective reductions.
Lithium tri-tert-butoxyaluminum hydride: a versatile reducing agent in organic chemistry, stands out for its high selectivity. The reagent targets specific functional groups, such as esters and acid chlorides, and converts them to aldehydes. It leaves other groups, like ketones, nitriles, and amides, mostly untouched. This selectivity comes from the steric hindrance created by the tert-butoxy groups. Chemists value this property when working with complex molecules that contain many reactive sites. The selectivity profile of lithium tri-tert-butoxyaluminum hydride makes it a top choice for reactions that require precise control. Other metal alkoxyaluminium hydrides do not always offer the same level of selectivity, so this reagent fills an important role in organic synthesis.
Chemists must handle lithium tri-tert-butoxyaluminum hydride: a versatile reducing agent in organic chemistry with care. This reagent reacts with water and moisture in the air. Contact with water can release flammable gases. Laboratories should store the reagent in tightly sealed containers under an inert gas like nitrogen or argon. Gloves and goggles protect the skin and eyes from accidental splashes. A fume hood helps keep vapors away from the user.
Spills require immediate cleanup with dry, inert materials. Water should never be used to clean up spills. Laboratories must keep fire extinguishers nearby because the reagent can ignite if it contacts moisture. Training in safe handling procedures helps prevent accidents. Chemists should always read the safety data sheet before using this reagent.
Tip: Always add lithium tri-tert-butoxyaluminum hydride: a versatile reducing agent in organic chemistry to the reaction mixture slowly. This step helps control the reaction and reduces the risk of sudden heat or gas release.
The price of lithium tri-tert-butoxyaluminum hydride: a versatile reducing agent in organic chemistry is higher than many common reducing agents. Specialty chemical suppliers offer this reagent in small quantities. Large-scale purchases may lower the cost per gram, but most labs use it for specific, high-value reactions. The cost reflects the complex synthesis and the need for careful packaging.
Availability depends on the supplier and region. Some countries have strict regulations for shipping and storing reactive chemicals. Researchers should check local rules before ordering. Most suppliers provide detailed information about storage and shelf life. The reagent remains stable for months if stored properly. Labs that use it often keep only small amounts on hand to reduce waste and safety risks.
Note: Plan purchases ahead of time to avoid delays in research projects. Some suppliers may need extra time to ship this reagent due to safety requirements.

Lithium aluminum hydride stands as one of the most powerful reducing agents in organic chemistry. Chemists often choose this reagent when they need to reduce a wide range of functional groups. LiAlH4 can convert esters, carboxylic acids, amides, nitriles, and even some less reactive compounds into alcohols. Its high reduction strength makes it very effective, but it also means less selectivity. This reagent does not discriminate well among different functional groups, so it often reduces everything it can.
A key difference between lithium aluminum hydride and lithium tri-tert-butoxyaluminum hydride lies in their selectivity and strength. The table below highlights these differences:
| Aspect | Lithium Tri-tert-butoxyaluminum Hydride (LTBA) | Lithium Aluminum Hydride (LiAlH4) |
|---|---|---|
| Reduction Strength | Lower reduction strength; less reactive | High reduction strength; highly reactive |
| Selectivity | More selective; reduces aldehydes and ketones selectively in presence of esters (which it reacts with very slowly) | Less selective; reduces nearly all reducible functional groups including esters |
| Steric Effects | Bulky reagent; favors conjugate (1,4) addition in α,β-unsaturated ketones | Less hindered; favors direct (1,2) addition in α,β-unsaturated ketones |
| Reaction Pathways | Does not typically undergo single-electron transfer pathways | Can undergo single-electron transfer pathways with some substrates |
| Functional Group Compatibility | Selective for sensitive groups due to lower reactivity | Broad reducing power, less discrimination among functional groups |
LiAlH4 reacts violently with water and must be handled with great care. Chemists always use dry solvents and special equipment when working with this reagent. The addition of hydride to carbonyl groups happens quickly, which can lead to over-reduction if not controlled. Because of its strength, LiAlH4 is not the best choice when a reaction requires selectivity or when sensitive groups are present.
Note: LiAlH4 is best for complete reductions but not for selective transformations.
Sodium borohydride offers a milder alternative to lithium aluminum hydride. This reagent reduces aldehydes and ketones easily, but it does not react with esters, carboxylic acids, or amides under normal conditions. Chemists often use sodium borohydride in water or alcohol solutions, which makes it safer and easier to handle than LiAlH4. Its selectivity allows for reductions in the presence of other functional groups that would react with stronger reagents.
Sodium borohydride works well for simple reductions, especially when only aldehydes or ketones need to be converted to alcohols. It does not require strict anhydrous conditions, so it fits well in teaching labs and routine organic synthesis. However, it cannot perform the same broad range of reductions as lithium aluminum hydride or some metal alkoxyaluminium hydrides.
Tip: Choose sodium borohydride for safe, selective reductions of aldehydes and ketones.
Diisobutylaluminum hydride, or DIBAL-H, serves as a versatile reagent for partial reductions. Chemists use DIBAL-H to reduce esters and nitriles to aldehydes without further reduction to alcohols. This reagent provides more control than lithium aluminum hydride, especially when the goal is to stop at the aldehyde stage. DIBAL-H works best at low temperatures, such as -78 °C, to prevent over-reduction.
DIBAL-H shares some features with other metal alkoxyaluminium hydrides, but its unique structure gives it special selectivity. It is less bulky than lithium tri-tert-butoxyaluminum hydride, so it can access more crowded sites on a molecule. However, DIBAL-H still requires careful handling because it reacts with moisture and can release flammable gases.
Chemists often choose DIBAL-H for reactions that need partial reduction or when working with sensitive functional groups. It offers a balance between strength and selectivity, making it useful for complex organic synthesis.
Note: DIBAL-H is ideal for converting esters and nitriles to aldehydes without over-reduction.
Organic chemists use a variety of reducing agents for different tasks. Some of these reagents offer unique selectivity or safety advantages. Red-Al and alane (AlH3) are two notable examples.
Red-Al, also known as sodium bis(2-methoxyethoxy)aluminum hydride, acts much like lithium aluminum hydride. It reduces a broad range of functional groups, including esters, carboxylic acids, and nitro groups. Many chemists prefer Red-Al for its safer handling and easier storage. Unlike LiAlH4, Red-Al dissolves in common organic solvents and does not react as violently with moisture. This feature makes it a practical choice for large-scale reductions.
Alane (AlH3) is less common but offers special selectivity. It can reduce carboxylic acids even when sensitive groups like halogens or nitro groups are present. This selectivity helps chemists avoid unwanted side reactions. Alane is less available than other reagents, so labs use it mainly for challenging reductions.
The table below compares these and other common reducing agents:
| Reducing Agent | Selectivity Profile | Key Features and Comparison to LTBA |
|---|---|---|
| Lithium Tri-tert-butoxyaluminum Hydride (LTBA) | Selective reduction, especially acid halides to aldehydes; steric bulk limits reactivity | Bulky tert-butoxy groups provide controlled, selective hydride delivery, reducing acid halides to aldehydes selectively |
| Lithium Aluminum Hydride (LiAlH4) | Strong, non-selective reducing agent | Reduces a wide range of functional groups indiscriminately; more reactive than LTBA |
| Sodium Borohydride (NaBH4) | Mild reducing agent | Less reactive and more selective than LiAlH4; generally milder than LTBA |
| DIBAL | Moderate selectivity | Useful for specific transformations like α,β-unsaturated carboxylic acids to allylic alcohols; more selective than LiAlH4 |
| Red-Al | Similar selectivity to LiAlH4 but safer and easier to handle | Shares LiAlH4’s broad reactivity but with improved handling and safety |
| Alane (AlH3) | Less commonly used; selective for carboxylic acids in presence of sensitive groups | Can reduce carboxylic acids without affecting halogen or nitro substituents; less available but useful in selective reductions |
Tip: Choosing the right reducing agent depends on the functional groups present and the desired product. Some reagents work best for selective reductions, while others excel in broad reactivity.
Some reductions require even milder conditions. Catalytic hydrogenation uses hydrogen gas and a metal catalyst to reduce double bonds or other unsaturated groups. This method offers high selectivity for certain transformations and avoids the use of reactive hydride reagents. However, hydrogenation may not work for all substrates, especially those sensitive to metal catalysts or high pressure.
Chemists must consider the strengths and weaknesses of each reagent. The choice depends on the substrate, the desired selectivity, and the safety requirements of the laboratory.

Chemists often choose lithium tri-tert-butoxyaluminum hydride for the reduction of acid halides, especially acid chlorides. This reagent stands out for its ability to stop the reaction at the formation of aldehydes. The bulky tert-butoxy groups slow down the reaction after the first hydride transfer. As a result, the aldehyde intermediate does not react further under these conditions. This selectivity is important in nucleophilic acyl substitution reactions, where controlling the product is key. When using LTBA, chemists can perform the reduction of acid chlorides at low temperatures, such as -78°C, to further improve selectivity. This method allows for the isolation of aldehydes without over-reduction to alcohols. The reduction of acid halides with LTBA is a reliable way to obtain sensitive aldehydes from reactive acid chlorides.
Tip: LTBA’s steric hindrance makes it ideal for selective nucleophilic acyl substitution, especially when the goal is to stop at the aldehyde stage.
Lithium aluminium hydride is a much stronger reducing agent. In the reduction of acid halides, this reagent does not stop at the aldehyde. Instead, it pushes the reaction all the way to the alcohol. When chemists use lithium aluminium hydride for the reduction of acid chlorides, they often see complete conversion to alcohols. Even if they try to use only one equivalent, the reaction produces a mixture of starting material, aldehyde, and alcohol. Over-reduction is very common and hard to avoid with this reagent. The nucleophilic acyl substitution mechanism proceeds quickly, and the aldehyde intermediate reacts further before it can be isolated. For those who want to obtain aldehydes, lithium tri-tert-butoxyaluminum hydride offers a better choice. The reduction of acid halides with lithium aluminium hydride is best when the goal is to make alcohols, not aldehydes.
Sodium borohydride shows some limitations in the reduction of acid halides. This reagent can reduce acyl chlorides because they are highly reactive. However, it cannot reduce carboxylic acids or esters. The reason lies in the nucleophilic acyl substitution pathway. Sodium borohydride forms a stable acyloxyborohydride intermediate with these less reactive substrates. This stable intermediate lowers the reducing power of sodium borohydride. As a result, the reduction of acid halides other than acyl chlorides does not proceed well. Chemists must consider these limitations when planning a reduction of acid halides. Sodium borohydride works best for simple, highly reactive acid chlorides, but not for less reactive acid halides or other carboxylic acid derivatives.
Sodium borohydride can reduce acyl chlorides due to their higher reactivity.
It cannot reduce carboxylic acids or esters because it forms a stable acyloxyborohydride intermediate.
This stable intermediate decreases the reducing power of sodium borohydride towards these substrates.
The limitation for other acid halides is related to the formation of stable intermediates and substrate reactivity.
Note: Always check the reactivity of your substrate before choosing sodium borohydride for the reduction of acid halides.
Diisobutylaluminum hydride, known as DIBAL-H, plays a special role in organic chemistry. Chemists use DIBAL-H when they want to control how far a reduction goes. This reagent can stop the reduction of acid chlorides at the aldehyde stage. DIBAL-H does not push the reaction all the way to the alcohol, which makes it different from stronger agents like lithium aluminum hydride.
DIBAL-H works well for the reduction of acid halides. The reagent delivers a hydride to the carbonyl carbon. The bulky isobutyl groups slow down the reaction. This control helps chemists isolate the aldehyde before it turns into an alcohol. The process follows a nucleophilic acyl substitution pathway. DIBAL-H adds a hydride to the acid chloride, forming an intermediate. The intermediate then loses a chloride ion, and after workup, the chemist gets the aldehyde.
Note: DIBAL-H must be used at low temperatures, often around -78°C. This step prevents over-reduction and keeps the reaction selective.
The reduction of acid chlorides with DIBAL-H is very useful in making sensitive aldehydes. Many chemists choose DIBAL-H for this reason. The reagent also works for the reduction of acid halides other than chlorides, but the selectivity may change depending on the substrate.
Here is a simple summary of how DIBAL-H compares to other reducing agents for acid halides:
| Reagent | Product from Acid Chloride | Over-Reduction Risk | Selectivity |
|---|---|---|---|
| DIBAL-H | Aldehyde | Low | High |
| LiAlH4 | Alcohol | High | Low |
| LTBA | Aldehyde | Very Low | Very High |
| NaBH4 | Alcohol (sometimes) | Moderate | Moderate |
DIBAL-H also uses the nucleophilic acyl substitution mechanism. The hydride attacks the carbonyl, and the leaving group departs. This pathway is common in the reduction of acid halides and acid chlorides. Chemists must control the temperature and the amount of DIBAL-H to avoid making too much alcohol.
DIBAL-H is best for partial reductions.
It gives chemists control over the product.
The reagent is less bulky than LTBA but still selective.
DIBAL-H remains a popular choice for the reduction of acid chlorides when the goal is to make aldehydes. Its selectivity and mild conditions make it valuable in both research and industry.
Lithium tri-tert-butoxyaluminum hydride (LTBA) works best in selective reductions where chemists want to stop at an intermediate product. The bulky tert-butoxy groups on LTBA slow down the delivery of hydride ions. This control helps chemists avoid over-reduction, especially in reactions where the product is more reactive than the starting material. For example, when reducing esters to aldehydes, LTBA gives better selectivity than lithium aluminum hydride or sodium borohydride. These other reagents often reduce the aldehyde further to an alcohol. LTBA’s unique structure makes it the preferred choice for these situations.
Tip: Use LTBA when you need to control the reduction and avoid making unwanted alcohols from esters or acid chlorides.
LTBA shows high chemoselectivity in complex molecules. It can reduce esters and acid chlorides to aldehydes while leaving other groups, such as ketones, nitriles, and amides, mostly untouched. This selectivity allows chemists to work with molecules that have many different functional groups. In contrast, lithium aluminum hydride reduces almost every group it encounters, which can cause problems in multi-step syntheses. Sodium borohydride, while safer, cannot reduce esters or acid chlorides under normal conditions. LTBA’s compatibility with sensitive groups makes it valuable in the synthesis of pharmaceuticals and natural products.
| Reagent | Reduces Esters | Stops at Aldehyde | Leaves Ketones Untouched | Suitable for Complex Molecules |
|---|---|---|---|---|
| LTBA | Yes | Yes | Yes | Yes |
| LiAlH4 | Yes | No | No | No |
| NaBH4 | No | No | Yes | Yes |
Chemists often choose LTBA for reactions that require careful control. In the synthesis of fragrances, LTBA helps produce aldehydes from esters without making unwanted alcohols. Researchers also use LTBA in asymmetric reductions, where controlling the product’s shape is important. For example, LTBA can help create chiral centers in molecules, which is useful in drug development. In some cases, chemists prefer hydrogenation or enantioselective catalytic reductions for even greater control, especially when working with double bonds or when a metal catalyst is needed. However, LTBA remains a top choice for selective reductions of esters and acid chlorides in sensitive or complex molecules.
Note: LTBA is not always the best option for every reduction. For simple reductions of aldehydes or ketones, sodium borohydride may offer a safer and cheaper alternative.
Lithium tri-tert-butoxyaluminum hydride is a specialty reagent. Most chemical suppliers carry it, but not all stock large quantities. Researchers often need to plan ahead when ordering this compound. Some suppliers require special permits or documentation before shipping. Shipping regulations may vary by country. Laboratories in remote areas may face longer delivery times. The cost can also fluctuate based on demand and supplier location. Many labs purchase only small amounts because of the price and storage requirements. Bulk orders may lower the cost per gram, but storage and safety become bigger concerns.
Tip: Always check local regulations and supplier policies before placing an order for reactive chemicals.
Proper storage keeps lithium tri-tert-butoxyaluminum hydride stable and safe. This compound reacts quickly with water and moisture in the air. Chemists store it in a cool place to slow down any unwanted reactions. They keep the container tightly closed to prevent exposure to air. A dry, well-ventilated area helps protect the reagent from humidity. Contact with water or moisture can cause dangerous reactions, so labs avoid any such exposure during storage. Many chemists handle the compound under an inert gas, such as nitrogen or argon, to shield it from moisture. Closed containers help maintain stability over time.
Store in a cool, dry, and well-ventilated area.
Keep containers tightly closed.
Avoid all contact with water or moisture.
Handle under inert gas when possible.
These steps help preserve the reagent’s effectiveness and reduce the risk of accidents. Proper storage also extends the shelf life, making the investment in this reagent more worthwhile.
Safety remains a top priority when working with lithium tri-tert-butoxyaluminum hydride. This reagent can ignite if it contacts water. Chemists always wear gloves, goggles, and lab coats to protect themselves. Work should take place in a fume hood to avoid inhaling any vapors. Labs keep fire extinguishers nearby in case of emergencies. Only trained personnel should handle this compound. Training includes learning how to add the reagent slowly to reaction mixtures and how to clean up spills using dry, inert materials. Water should never be used for cleanup. Labs also post clear instructions and emergency procedures in visible locations.
| Safety Measure | Purpose |
|---|---|
| Gloves and goggles | Protect skin and eyes |
| Fume hood | Prevent inhalation of vapors |
| Inert gas handling | Reduce risk of moisture exposure |
| Fire extinguisher | Prepare for accidental ignition |
| Training | Ensure safe handling and emergency response |
Note: Reviewing the safety data sheet before use helps everyone stay informed and prepared.
Chemists must consider the environmental impact of lithium tri-tert-butoxyaluminum hydride (LTBA) before using it in the laboratory. This reagent, like many strong reducing agents, can create waste that harms the environment if not handled properly. LTBA contains aluminum and lithium, both of which can cause problems if released into soil or water.
Waste Generation and Disposal
LTBA produces hazardous waste during reactions and cleanup. The byproducts often include aluminum salts, lithium compounds, and organic residues. These substances can pollute water sources and harm aquatic life. Laboratories must collect all waste from LTBA reactions in special containers. They should never pour leftover chemicals down the drain or throw them in regular trash.
Tip: Always follow local regulations for hazardous waste disposal. Many cities have special programs for chemical waste from laboratories.
Air and Water Hazards
LTBA reacts with water to release flammable gases. If spilled, it can create dangerous fumes that pollute the air. Proper ventilation and spill control help reduce these risks. Chemists should use fume hoods and keep LTBA away from sinks and drains. Even small amounts of LTBA can cause problems if they reach the environment.
Resource Use and Sustainability
The production of LTBA requires energy and raw materials. Mining for lithium and aluminum uses large amounts of water and can damage natural habitats. Factories that make LTBA may also produce greenhouse gases. These factors increase the environmental footprint of this reagent.
Safer and Greener Alternatives
Some chemists look for greener reducing agents to lower their environmental impact. Sodium borohydride and catalytic hydrogenation often create less hazardous waste. These alternatives may not always match LTBA’s selectivity, but they offer safer disposal and lower toxicity.
| Reducing Agent | Hazardous Waste | Air/Water Risk | Greener Option? |
|---|---|---|---|
| LTBA | High | High | No |
| Sodium Borohydride | Moderate | Moderate | Yes |
| Catalytic Hydrogenation | Low | Low | Yes |
Best Practices for Reducing Environmental Impact
Use only the amount of LTBA needed for each reaction.
Collect and label all waste for proper disposal.
Train all lab members in safe handling and spill response.
Consider alternative reagents when possible.
Note: Responsible use and disposal of LTBA protect both people and the planet. Chemists play a key role in reducing the environmental impact of their work.
Chemists often judge a reducing agent by how well it completes a reaction. Some agents work quickly and reduce many types of chemical bonds. Others act more slowly or only reduce certain groups. The table below shows how each common reducing agent performs in terms of effectiveness:
| Reducing Agent | Effectiveness (General Scope) | Typical Use Cases |
|---|---|---|
| LTBA | Moderate | Selective reductions, esters to aldehydes |
| LiAlH4 | Very High | Broad reductions, strong reducing power |
| NaBH4 | Moderate | Aldehydes and ketones |
| DIBAL-H | High (for partial reductions) | Esters/nitriles to aldehydes |
| Red-Al | High | Similar to LiAlH4, safer handling |
| Alane (AlH3) | Moderate | Carboxylic acids, special cases |
Tip: LiAlH4 stands out for its strong reducing power, but LTBA and DIBAL-H offer more control for specific reactions.
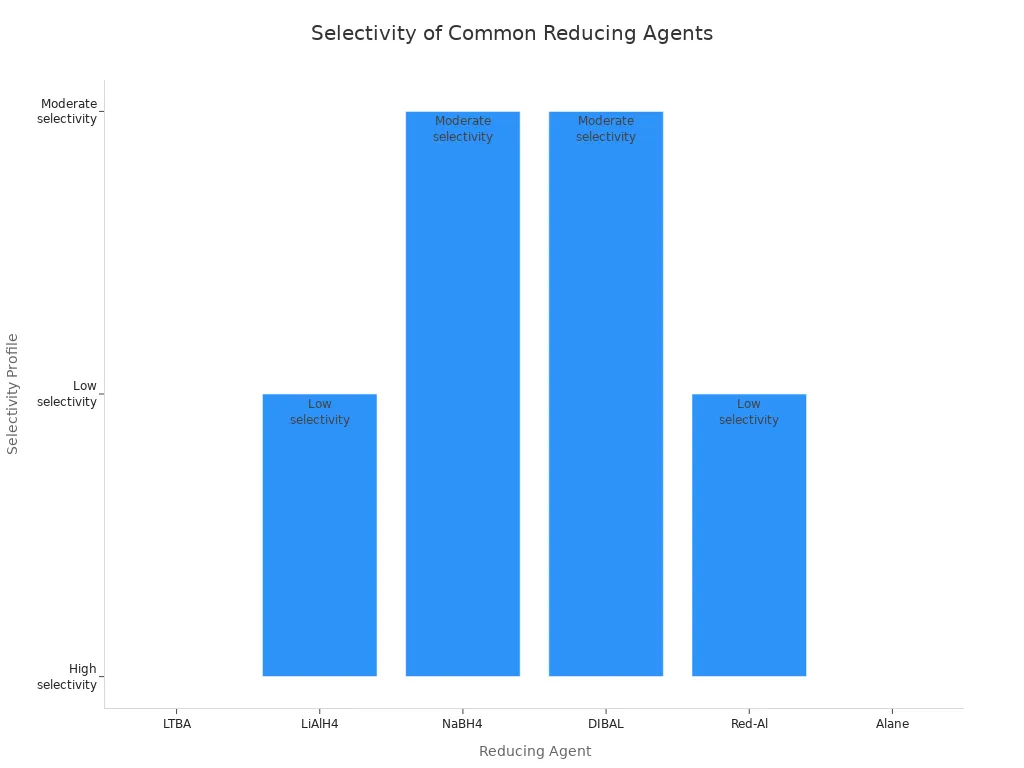
Selectivity means a reducing agent can target one group in a molecule without touching others. This property helps chemists avoid unwanted side products. LTBA and DIBAL-H both show high selectivity, especially when making aldehydes from esters or acid chlorides. LiAlH4, on the other hand, reduces almost everything it can.
| Reducing Agent | Selectivity Level | Best For |
|---|---|---|
| LTBA | Very High | Stopping at aldehyde, chemoselective work |
| LiAlH4 | Low | Complete reductions |
| NaBH4 | Moderate | Aldehydes/ketones, leaves esters untouched |
| DIBAL-H | High | Partial reductions, stops at aldehyde |
| Red-Al | Low | Broad reductions, less selectivity |
| Alane (AlH3) | Moderate | Carboxylic acids with sensitive groups |
Selective agents like LTBA help chemists isolate products without extra purification steps.
Safety is a top concern in any laboratory. Some reducing agents react violently with water or air. Others are easier and safer to handle. Chemists must always follow strict safety rules, but some reagents pose more risks than others.
| Reducing Agent | Safety Risk Level | Key Hazards |
|---|---|---|
| LTBA | Moderate-High | Reacts with water, flammable |
| LiAlH4 | High | Violent with water, fire risk |
| NaBH4 | Low-Moderate | Safer, but still needs care |
| DIBAL-H | High | Flammable, reacts with moisture |
| Red-Al | Moderate | Safer than LiAlH4, still reactive |
| Alane (AlH3) | High | Flammable, special handling |
Note: Always wear gloves, goggles, and use a fume hood when working with strong reducing agents.
Cost often plays a big role when chemists choose a reducing agent. Some reagents cost much more than others because of how they are made or how hard they are to get. Labs must think about both the price per gram and how much reagent they need for each reaction.
| Reducing Agent | Relative Cost | Notes on Cost |
|---|---|---|
| LTBA | High | Specialty item, higher price, used in small amounts |
| LiAlH4 | Moderate | Widely available, reasonable price |
| NaBH4 | Low | Inexpensive, often bought in bulk |
| DIBAL-H | High | Expensive, used for special tasks |
| Red-Al | Moderate | Safer than LiAlH4, price is similar |
| Alane (AlH3) | High | Rare, hard to find, costly |
LTBA and DIBAL-H both cost more than common agents like sodium borohydride or lithium aluminum hydride. These specialty reagents often come in small bottles and are used only when their selectivity is needed. Sodium borohydride is the most affordable option for many labs. It works well for simple reductions and is easy to buy in large amounts.
Tip: Chemists can save money by choosing the least expensive reagent that still meets their needs. They should also consider the cost of waste disposal and safety equipment.
Some labs may pay more for a reagent if it saves time or improves the yield of a valuable product. In research and industry, the total cost includes not just the price of the chemical, but also the cost of handling, storage, and cleanup.
Practicality means how easy it is to use a reducing agent in real lab work. Some reagents need special equipment or strict safety steps. Others are simple to use and store.
Key factors for practicality:
Ease of handling: Can students or new chemists use it safely?
Storage needs: Does it need a special container or inert gas?
Reaction conditions: Does it work at room temperature or need cooling?
Waste disposal: Is it easy to get rid of safely?
| Reducing Agent | Practicality Level | Practical Notes |
|---|---|---|
| LTBA | Moderate | Needs dry conditions, careful handling |
| LiAlH4 | Low | Requires anhydrous setup, reacts with water |
| NaBH4 | High | Easy to use, works in water or alcohol |
| DIBAL-H | Moderate | Needs low temperatures, sensitive to moisture |
| Red-Al | Moderate | Easier than LiAlH4, but still needs care |
| Alane (AlH3) | Low | Rarely used, special handling required |
Sodium borohydride stands out for its practicality. Most students can use it with basic training. It works in water or alcohol and does not need special storage. Lithium aluminum hydride and LTBA both need dry conditions and careful handling. DIBAL-H often needs cooling with dry ice, which adds extra steps.
Note: Chemists should match the reagent to their lab’s equipment and skill level. Choosing a practical reagent can save time and reduce accidents.
Some reagents also create hazardous waste that needs special disposal. Labs should plan for this before starting any reaction. Practicality helps chemists finish their work safely and efficiently.
Lithium tri-tert-butoxyaluminum hydride stands out for selective and stereoselective reductions. Recent studies show it delivers quantitative yields and complete diastereoselectivity in sulfinylimine reductions, outperforming other agents:
| Reagent | Yield (%) | Diastereomeric Ratio (dr) |
|---|---|---|
| NaBH4 | 100 | 71:29 |
| DIBAL | 100 | 100:0 |
| LTBA | 100 | 100:0 |
Chemists should match the reducing agent to the substrate and desired outcome. Considering effectiveness, selectivity, safety, cost, and practicality ensures the best results. For complex reductions, consulting experts or review articles can provide valuable guidance.
LTBA offers higher selectivity and milder reactivity than LiAlH4. Chemists use LTBA to stop reductions at the aldehyde stage, while LiAlH4 often reduces compounds all the way to alcohols.
Students should only use LTBA under close supervision. The reagent reacts with water and can ignite. Proper training, gloves, goggles, and a fume hood are essential for safe handling.
LTBA reduces esters and acid chlorides to aldehydes with high selectivity. It usually leaves ketones, nitriles, and amides unchanged. This selectivity helps chemists target specific groups in complex molecules.
Labs should store LTBA in tightly sealed containers under an inert gas, such as nitrogen. The storage area must stay cool and dry. Moisture and air exposure can cause dangerous reactions.
Yes, LTBA costs more than common agents like sodium borohydride or lithium aluminum hydride. Labs often use LTBA in small amounts for special reactions that require its selectivity.
Chemists should cover spills with dry, inert material and avoid water. They must clean up in a fume hood and dispose of waste in a hazardous waste container. Always follow the lab’s emergency procedures.
Catalytic hydrogenation and sodium borohydride offer greener options for some reductions. These alternatives create less hazardous waste and pose fewer environmental risks, but may not match LTBA’s selectivity.
LTBA’s high selectivity allows chemists to reduce specific groups without affecting others. This control is important in pharmaceutical synthesis, where purity and precise structure matter.