Views: 0 Author: Site Editor Publish Time: 2025-08-11 Origin: Site









You can achieve high selectivity in organic reactions by understanding how L-selectride improves reaction selectivity in organic chemistry. This reagent stands out because of its large size and unique electronic properties, which contribute to exceptional chemoselectivity. For instance, when using L-selectride at -78 °C, you can obtain a cis:trans isomer ratio greater than 20:1, whereas other hydride reagents typically only reach up to 3.8:1. The chart below highlights this significant improvement in both chemoselectivity and yield.
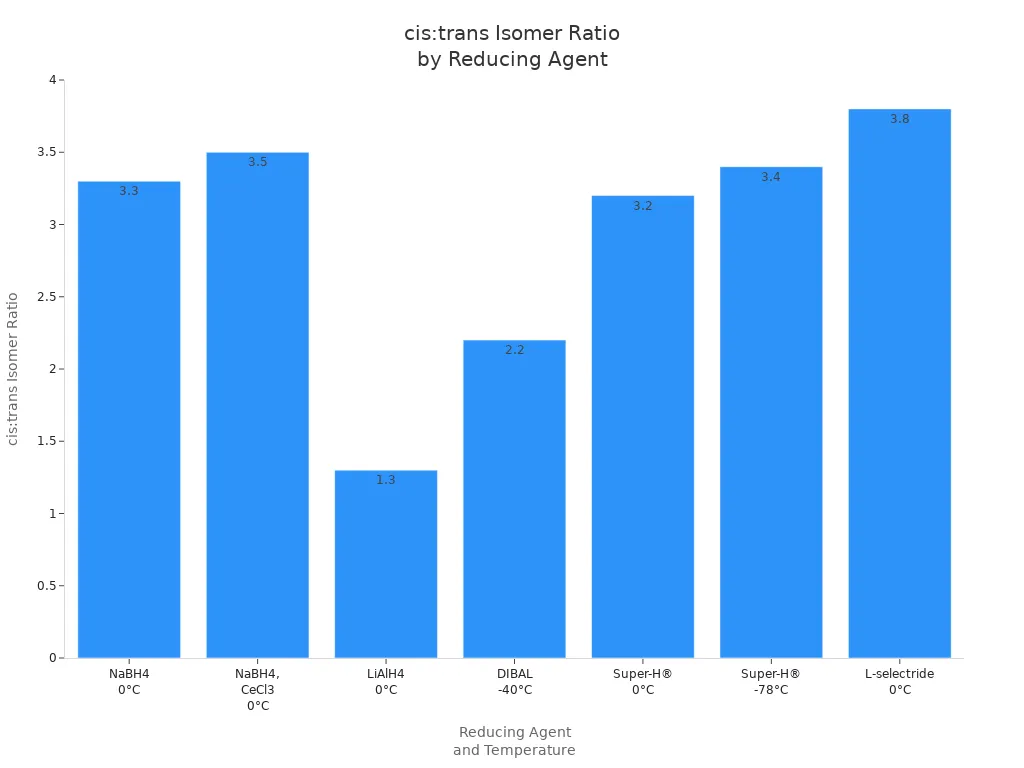
By leveraging how L-selectride improves reaction selectivity in organic chemistry, you gain cleaner results and greater control over your product’s structure. Using proven strategies, you can maximize chemoselectivity in your own experiments. Try these approaches to experience firsthand how L-selectride enhances reaction selectivity in organic chemistry.
Steric effects help you control chemoselectivity in organic reactions. L-selectride is big and bulky. Its large size keeps it away from crowded spots on a molecule. When you reduce a cyclic ketone, L-selectride attacks the side with more space. This gives mostly the cis isomer. Smaller hydride reagents, like NaBH4 or LiAlH4, make more trans product. The table below shows how different reagents change the results when reducing a cyclic ketone:
| Reagent | % trans | % cis |
|---|---|---|
| NaBH4 | ~74 | ~26 |
| LiAlH4 | ~76 | ~24 |
| Borane (B2H6) | ~69 | ~31 |
| Lithium tri(tert-butoxy)aluminum hydride | ~56 | ~44 |
| L-Selectride | ~1.5 | ~98.5 |
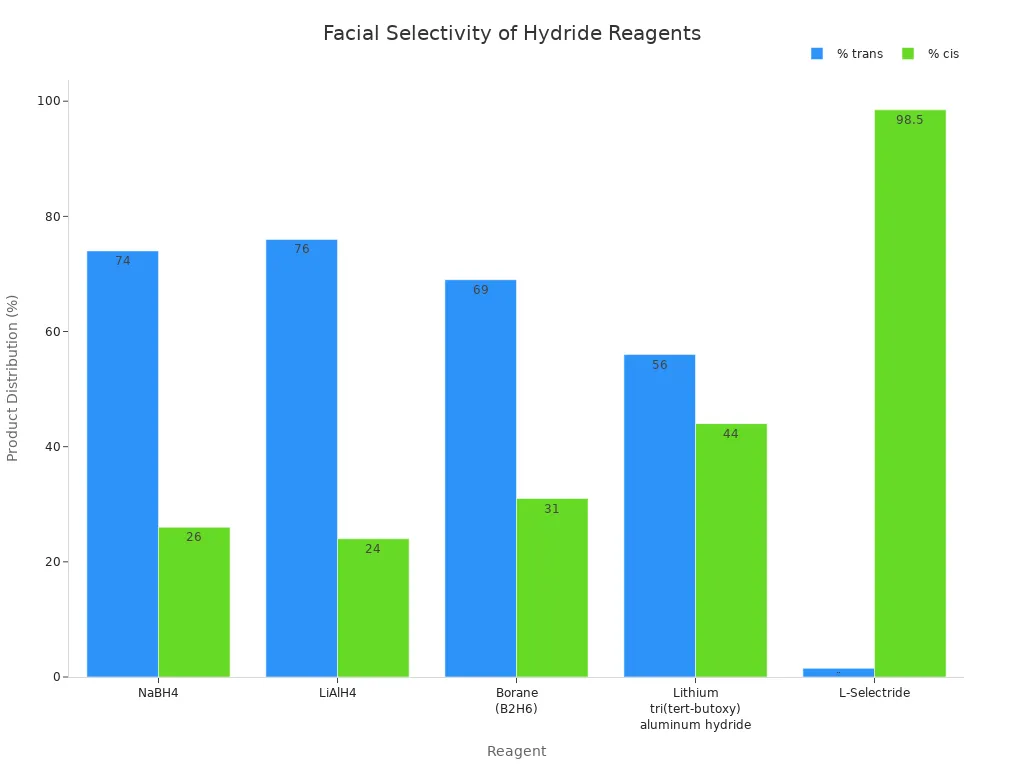
L-selectride gives almost only cis product when reducing a ketone. This happens because the big reagent stays away from crowded sides. If the ketone has large groups, this effect is even stronger.
Electronic effects also change chemoselectivity. L-selectride likes to reduce a ketone more than an ester. Its electronic properties make it react faster with carbonyl groups. In α,β-unsaturated ketones, L-selectride often adds to the β-carbon (1,4-addition). It does this instead of adding to the carbonyl carbon (1,2-addition). Smaller hydrides can give a mix of products. You can use this to get chemoselectivity in tricky molecules. Chemoselectivity depends on the electronic nature of the carbonyl and if a ketone is present.
L-selectride’s bulky size and electronic features help you pick the right group to reduce.
You can get chemoselectivity even with both an aldehyde and a ketone, but L-selectride usually reduces the ketone first.
The shape of your substrate also changes chemoselectivity. If a ketone has big groups, L-selectride attacks the less crowded side. In steroids, a ketone at the 3-position can be reduced even if there is another ketone somewhere else. The result depends on what is around the ketone. For example, if you reduce a steroid with no extra groups on the A or B rings, you get mostly β-hydroxyl products. If you add big groups at certain spots, you get more α-alcohols.
When reducing an aldehyde with L-selectride, selectivity depends on the shape and crowdedness of the substrate.
Metal chelation can hold the substrate in one shape, making chemoselectivity even better.
You can use these ideas to plan reductions of both aldehydes and ketones in tricky molecules.
Tip: Always look for a ketone and check the groups around it if you want the best chemoselectivity in your reaction.

L-selectride helps you pick which group to react in a molecule. Chemoselectivity means you can choose one group to change, even if others could react too. L-selectride is special because it likes to reduce groups that are not crowded. If your molecule has both a ketone and an aldehyde, L-selectride usually reduces the ketone first. This happens because L-selectride is big and cannot fit into tight spaces.
Chemoselectivity is important when you want to avoid making extra products. Cleaner reactions mean you spend less time cleaning up. L-selectride is great for reducing α,β-unsaturated compounds. It often gives you the product from 1,4-addition, while other reagents might give a mix of products. This means you get more of the product you want.
Chemoselective reactions help you work with molecules that are easy to break. If your molecule has both an aldehyde and a ketone, L-selectride lets you reduce just one. This control helps you make complex molecules in fewer steps. You can trust L-selectride to reduce only the group you want and leave the others alone.
Tip: Always look for both aldehyde and ketone groups in your molecule. L-selectride helps you pick which one to reduce first, so your work is faster.
L-selectride does more than just pick which group to reduce. It also helps you choose the shape of your product. When you use L-selectride, you can control which side of the molecule gets reduced. This is important for making molecules with the right 3D shape.
For example, when you reduce 4-tert-butylcyclohexanone, L-selectride gives a cis:trans ratio of 20:1. This means almost all the product is the cis isomer. Other reagents, like sodium borohydride, only give a ratio of 2.4:1. This shows L-selectride is better at picking the shape you want.
The table below shows more examples. It lists the ratios and excesses you can get with L-selectride in different cases:
| Substrate/Reaction Condition | Reductant | Solvent | Temperature | Diastereomeric Ratio (dr) or Enantiomeric Excess (ee) |
|---|---|---|---|---|
| 2-substituted 3-hydroxypiperidines (14a) | L-Selectride | THF | −78°C | dr = 6:1 anti/syn |
| Same substrate (14a) | L-Selectride | CH2Cl2/THF | −78°C | dr > 19:1 anti/syn |
| Diol anti-9a (from L-Selectride reduction) | L-Selectride | THF/CH2Cl2 | N/A | dr = 25:1; ee = 32% (after Appel reaction, racemization noted) |
| Piperidine trans-11a (hydrochloride salt, optimized conditions) | L-Selectride | N/A | Low temperature | ee ≥ 99%, single diastereomer |
| 4-tert-butylcyclohexanone reduction | L-Selectride | N/A | N/A | cis:trans = 20:1 (favoring cis) |
| α-Keto esters (3a) | L-Selectride | EtOH | −78°C | dr = 85:15 (4/5 ratio) |
| α-Keto esters (3a) + ZnCl2 | L-Selectride/ZnCl2 | EtOH | −78°C | dr > 99:1 |
| α-Keto esters (3b) | L-Selectride | EtOH | −78°C | dr = 94:6 |
| α-Keto esters (3b) + ZnCl2 | L-Selectride/ZnCl2 | EtOH | −78°C | dr > 99:1 |
| α-Keto esters (3c) | L-Selectride | EtOH | −78°C | dr = 94:6 |
| α-Keto esters (3c) + ZnCl2 | L-Selectride/ZnCl2 | EtOH | −78°C | dr = 98:2 |
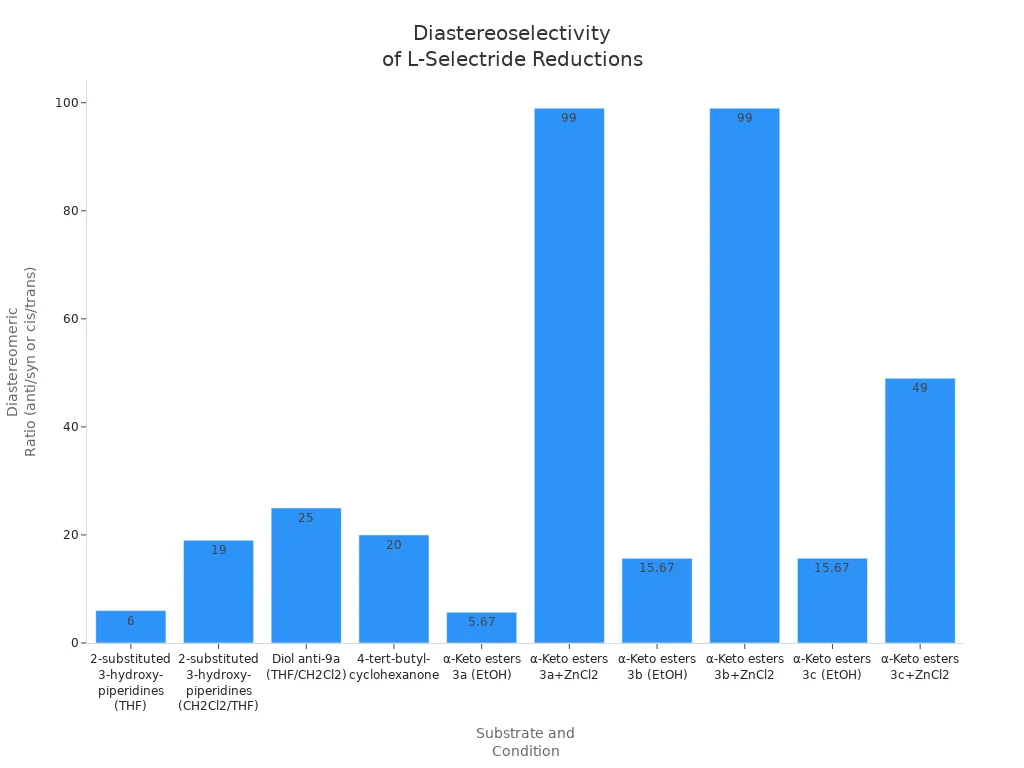
L-selectride alone gives good control over the product’s shape. You can get even better results by adding other chemicals. Sometimes, you can get a ratio higher than 25:1 or an excess of 99%. This much control is rare with other reagents.
You can make L-selectride work even better by adding other chemicals. ZnCl2 is one of the best helpers. When you add ZnCl2, it sticks to the carbonyl oxygen in your molecule. This holds the molecule in one shape. L-selectride can then only attack from one side, so you get more of the product you want.
Studies show ZnCl2 changes the results of the reaction. For example, if you reduce α-keto esters with L-selectride and ZnCl2, you can get a ratio higher than 99:1. Without ZnCl2, the ratio might only be 85:15 or 94:6. The chart below shows how ZnCl2 helps:
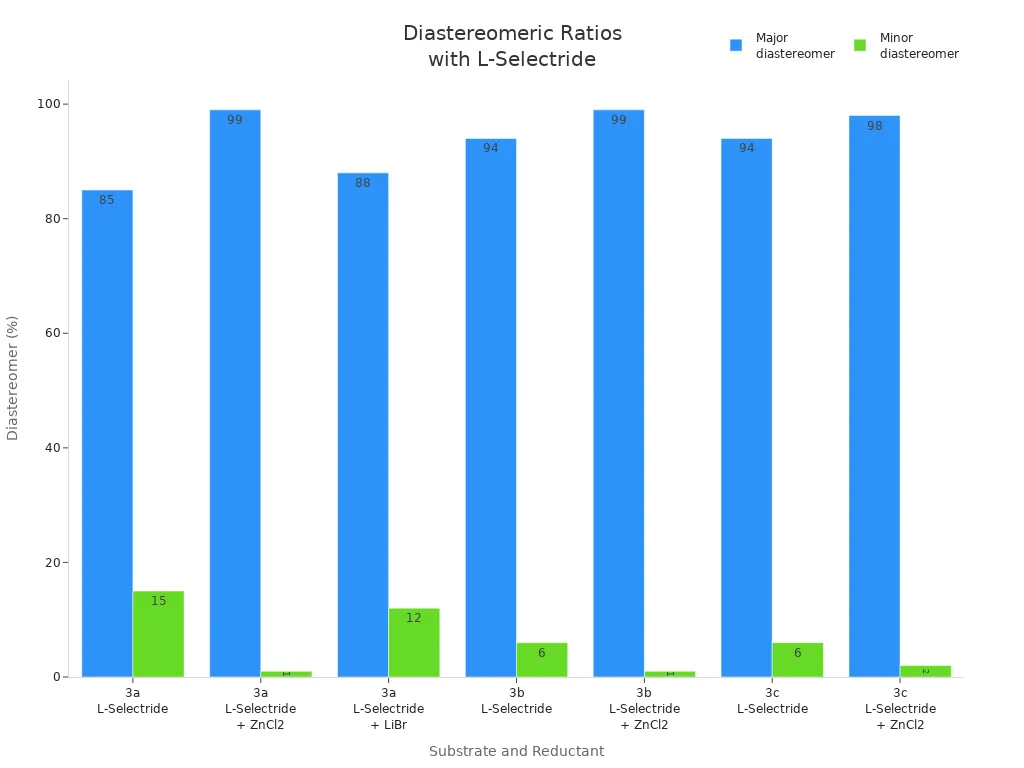
ZnCl2 works by holding the molecule in an s-cis shape. This blocks L-selectride from one side, so you get more of the product you want. You can use other helpers, like LiBr, but ZnCl2 works best for these reactions.
Note: Always use clean L-selectride and glassware. Dirt can lower selectivity and make your results less good. Good results depend on pure chemicals and careful work.
Now you know how L-selectride helps you control reactions in organic chemistry. By picking the right helpers and conditions, you can get chemoselectivity and stereoselectivity in your own reactions. This lets you reduce aldehyde, ketone, and other groups with confidence. You can also use L-selectride for other reactions that need careful control.
You can get better chemoselectivity by picking the right substrate. Look for molecules with oxygen atoms, like benzyloxy groups or carbonyls. These groups help lithium chelation. Lithium chelation guides the hydride to the best spot. This makes chemoselectivity much stronger. For example, a benzyloxy group often gives high syn-diastereoselectivity and good yields. The table below shows how different groups change yield and selectivity:
| Substituent (R) | Yield (%) | syn/anti Ratio |
|---|---|---|
| CH3 | 93 | 6:1 |
| C2H5 | 97 | 7:1 |
| n-C4H9 | 97 | 7:1 |
| n-C5H11 | 95 | 23:2 |
| n-C8H17 | 98 | 23:2 |
| n-C12H25 | 85 | 9:1 |
| n-C16H33 | 83 | 7:1 |
| i-Bu | 92 | 3:1 |
| Ph | 81 | 3:1 |
| Bn | 92 | 11:2 |
| PhCH2CH2 | 82 | 7:2 |
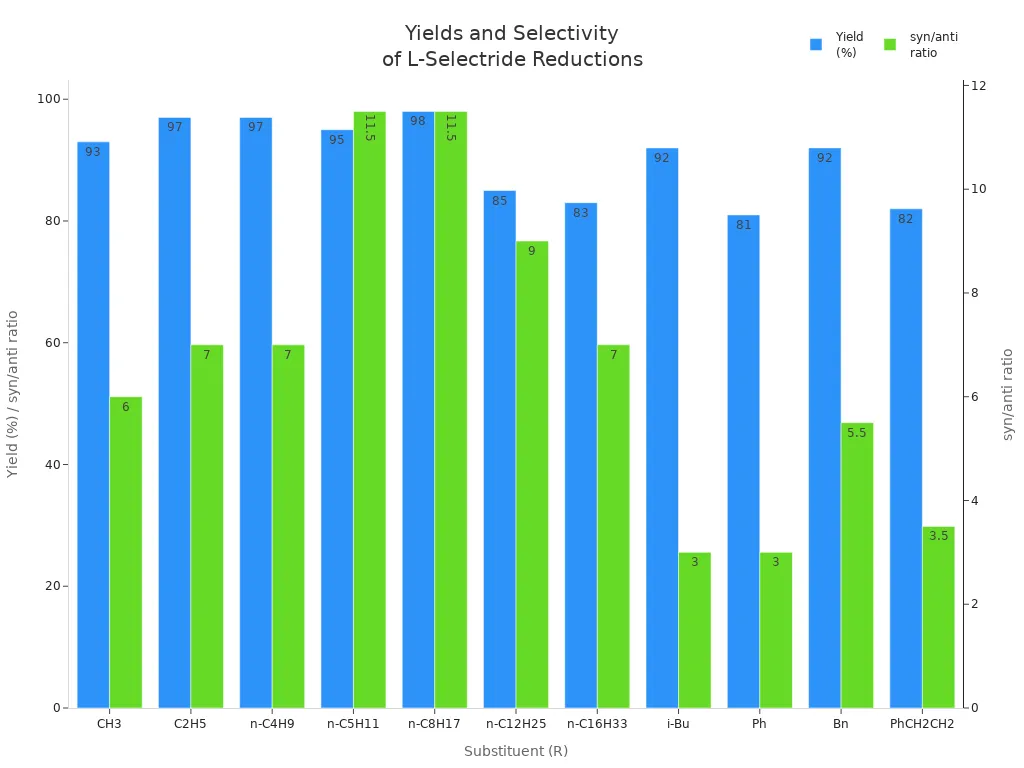
Try not to use substrates without coordinating groups. These usually give poor chemoselectivity. If your molecule has both a ketone and an aldehyde, L-selectride will usually reduce the ketone first. This helps you plan for making complex molecules.
You can get better chemoselectivity by changing reaction conditions. Use dry solvents like THF or dichloromethane. Keep the temperature low, like -78°C. This slows the reaction and gives you more control. Lower temperatures help you get better chemoselectivity. This is important when both a ketone and an aldehyde are present. Use the right amount of substrate. Too much or too little can lower chemoselectivity.
Tip: Always check your glassware for water or dirt. Clean tools help you keep chemoselectivity high.
You can use smart ways to add L-selectride and stop the reaction. Add L-selectride slowly to your substrate. This helps you avoid over-reduction and keeps chemoselectivity high. Watch the reaction closely. Use TLC or NMR to check when the aldehyde or ketone is gone. When you finish, stop the reaction with acetic acid (HOAc) or dilute HCl. These help you keep the right stereochemistry and avoid side products.
Add L-selectride drop by drop for better chemoselectivity.
Watch the reaction so you can stop at the right time.
Use HOAc or HCl to stop the reaction for the best results.
You can use these tips to get the most from L-selectride. Careful choices and good habits help you get high chemoselectivity, even with hard molecules that have both a ketone and an aldehyde.
L-selectride can help you get very good selectivity. But there are some mistakes that can make your yield lower or give you the wrong product. If you know about these problems, you can stop them and get better results.
Over-reduction happens if you use too much L-selectride or wait too long. You might get side products you do not want or lose your main product.
Signs of over-reduction:
The mixture gets cloudy or changes color.
You see extra spots on your TLC plate.
Your yield is lower than you want.
Tip: Add L-selectride slowly and check your reaction often. Use TLC or NMR to see how the reaction is going. Stop the reaction as soon as your starting material is gone.
L-selectride does not work well with every functional group. Some groups can react in ways you do not want. For example, nitro groups, acid chlorides, or some esters can cause problems.
Common problems:
L-selectride reduces groups you want to keep safe.
Sensitive groups break down or make side products.
Water or alcohols in your substrate can destroy L-selectride.
| Functional Group | Risk with L-selectride | Solution |
|---|---|---|
| Nitro | Over-reduction | Protect or avoid |
| Ester | Partial reduction | Use milder conditions |
| Alcohol | Destroys reagent | Dry your substrate |
Note: Always check your molecule for groups that might react. Make sure your glassware and solvents are dry to keep L-selectride working.
If you do not watch your reaction, you can miss the right time to stop. This can cause over-reduction or leave your reaction unfinished.
Common mistakes:
You forget to check TLC or NMR.
You trust the clock instead of checking the chemistry.
You do not stop the reaction at the right time.
Alert: Always set a timer and check your reaction every 10–15 minutes. Stop the reaction as soon as you see the product you want.
If you watch out for these mistakes, you can use L-selectride with confidence. Careful work helps you get the best selectivity and yield every time.

L-selectride helps you control how a ketone is reduced in big molecules. Chemists used it when making tricyclic marine alkaloids. They needed to change the way an alcohol was arranged. L-selectride was used to reduce a ketone and flip the alcohol’s shape. This shows L-selectride helps you get the right product, even in large, complex molecules. Because L-selectride is bulky, it goes to the less crowded side of a ketone. This helps you avoid unwanted reactions, like reducing an aldehyde by mistake. Picking L-selectride is important when you want just one product from many possible ones. If you want good control when reducing a ketone, L-selectride is a tool you can trust.
L-selectride can help you make more of one enantiomer than the other. This is called asymmetric hydrogenation. It is important for making medicines and natural products. The table below shows how L-selectride works in these reactions with α,β-unsaturated carboximide derivatives:
| Reaction Substrate | Conditions | Product | Diastereoselectivity | Enantioselectivity (ee) |
|---|---|---|---|---|
| α,β-unsaturated carboximide (R-oxazolidinone) | L-Selectride, THF, -78°C | Product 7 | 95:5 | >96% |
| α,β-unsaturated carboximide (S-oxazolidinone) | L-Selectride, THF, -78°C | Product 10 | >96:4 | >96% |
You can see that L-selectride gives high control in these reactions. You get over 96% of one enantiomer. This is hard to do with other reagents. If you want to make chiral molecules, try using L-selectride for asymmetric hydrogenation. This method works well for many molecules with a ketone or similar group.
L-selectride is useful for making natural products with high yield and selectivity. Chemists use it to reduce ketones in ring-shaped molecules, like tetrahydropyran rings. In one study, L-selectride reduced 6-alkyl-5-benzyloxy-6-hydroxy-2-piperidinones at cold temperatures. The yields were between 81% and 98%, and the syn/anti ratios went up to 23:2. You can see these results in the table below:
| Substrate | Temperature | Yield (%) | syn/anti Ratio |
|---|---|---|---|
| 6-alkyl-5-benzyloxy-6-hydroxy-2-piperidinone | -20°C to RT | 81-98 | 3:1 to 23:2 |
L-selectride helps you build hard molecules like Peloruside A, Epothilones, and Mycalamide A. You can use it for steps that need asymmetric hydrogenation. The bulky size lets you pick which side of the ketone gets reduced. This gives you good results and high yields. If you want to make natural products with many rings and chiral centers, L-selectride and asymmetric hydrogenation give you the control you need.
Tip: For best results, always use low temperatures and dry THF when using L-selectride for asymmetric hydrogenation and ketone reduction.
If you want better selectivity, use L-selectride in your reaction. This reagent helps you get more product and control its shape. The table below compares L-selectride to other hydride reagents:
| Feature | L-selectride | Sodium Borohydride | DIBAL-H | Schwartz’s Reagent |
|---|---|---|---|---|
| Yield | High (83%) | Lower | Lower | Moderate (77%) |
| Diastereoselectivity | 1:10.5 dr | Poor | Better | >20:1 dr |
Try using L-selectride in your next lab. You can learn more by reading chemistry books or looking up science journals.
L-selectride helps you reduce ketones, aldehydes, and some esters. It lets you make mostly one product in your reaction. This means you get better selectivity than with other reagents.
Keep L-selectride in a cool and dry spot. Store it under nitrogen gas so air does not get in. Make sure to keep it away from water and always use dry glassware.
No, you cannot use it with every group. L-selectride reacts with some groups like nitro or acid chlorides. Always check your molecule for these groups before you start.
L-selectride is big and bulky. Its size makes it go to less crowded places on a molecule. This gives you more control over which product you get. Smaller hydride reagents do not work as well for this.
Look at your glassware to see if it is dry. Make sure your L-selectride is not old. Watch your reaction using TLC or NMR. Try using a lower temperature or a different solvent to help your reaction work better.