Views: 0 Author: Site Editor Publish Time: 2025-08-05 Origin: Site









Recent breakthroughs in lithium borohydride have redefined its potential as an energy storage system. Researchers have demonstrated that composite modifications, such as combining LiBH4 with Li3AlH6, significantly lower dehydrogenation temperatures while improving kinetics and hydrogen release. Nanoconfinement within mesoporous carbon aerogels enables stable hydrogen cycling at much lower temperatures. Digital alloy design now allows precise control over intermediate phase formation, enhancing reversibility. These advances drive innovation and reinforce the Lithium Borohydride: Key Role in Hydrogen Storage Solutions, making this material increasingly relevant for practical applications.
Researchers in 2025 have made significant progress by developing new composite materials for lithium borohydride hydrogen storage. These composites combine lithium borohydride with other substances to improve hydrogen release and storage properties.
Scientists have discovered that combining lithium borohydride with materials like fluorographite creates powerful synergistic effects. These effects lower the temperature needed for hydrogen release and speed up the process. The interaction between lithium borohydride and the added material changes the way hydrogen atoms move and bond, making storage and release more efficient.
The LiBH4–fluorographite composite starts releasing hydrogen at around 180 °C, much lower than pure lithium borohydride.
This composite can release about 7.2–7.4% hydrogen by weight below 200 °C in just seconds.
Nanoscale lithium borohydride spots, about 90 nanometers wide, form on the fluorographite surface, which helps hydrogen move more easily.
Studies using X-ray diffraction and infrared analysis show that lithium borohydride stays stable after processing and interacts with fluorographite during hydrogen release.
The composite can partially store hydrogen again after use, showing improved reversibility.
The improved performance comes from the nano-sized structure and a helpful reaction between lithium borohydride and fluorographite.
These composite modifications address several key challenges. They lower the temperature needed for hydrogen release, which makes the process safer and more energy-efficient. The faster hydrogen release means that fuel cells and other devices can operate more quickly. Improved reversibility allows the material to be used again and again, which is important for real-world applications. These gains mark a major step forward in making lithium borohydride a practical choice for hydrogen storage.
Nanoconfinement has emerged as a game-changer in 2025. By trapping lithium borohydride inside tiny pores of materials like mesoporous carbon aerogels, researchers have achieved stable hydrogen cycling at much lower temperatures. The confined space changes the way lithium borohydride behaves, making it easier to release and absorb hydrogen. This method also prevents the particles from clumping together, which keeps the material working well over many cycles. Nanoconfinement directly addresses the problems of high release temperatures and slow reaction speeds.
Digital alloy design uses computer modeling and machine learning to create new lithium borohydride-based materials. Scientists can now predict how different combinations of elements will behave before making them in the lab. This approach speeds up the discovery of new alloys that have better hydrogen storage properties. Digital design helps control the formation of intermediate phases, which improves the reversibility and cycling stability of the material. These technological advancements allow for precise tuning of hydrogen storage systems, making them more reliable and efficient.
Note: The breakthroughs in 2025 have solved many of the old problems with lithium borohydride, such as high release temperatures, slow kinetics, and poor regeneration. These improvements bring hydrogen storage closer to everyday use in clean energy systems.

Lithium borohydride: key role in hydrogen storage solutions stands out because it can store a high level of hydrogen. This material holds up to 18.3% hydrogen by weight, which ranks among the highest for solid-state hydrogen storage solutions. Such a high hydrogen content means that smaller amounts of lithium borohydride can store large volumes of hydrogen. This property makes it attractive for applications where space and weight matter, such as portable fuel cells and transportation. Many researchers consider lithium borohydride: key role in hydrogen storage solutions a leading candidate for next-generation hydrogen storage solutions because of its impressive hydrogen storage properties.
Lithium borohydride: key role in hydrogen storage solutions offers a significant lightweight advantage. Its high gravimetric hydrogen capacity means it stores more hydrogen per unit of mass than most other materials. The volumetric capacity reaches 121 kg of hydrogen per cubic meter, which supports efficient storage in compact spaces. Scientists have found that mechanical treatments, such as stretching or compressing the material, can improve its performance. These treatments reduce the energy needed to release hydrogen, bringing the operating temperature closer to what fuel cells require. Density functional theory calculations and experiments confirm that lithium borohydride: key role in hydrogen storage solutions combines a lightweight structure with tunable properties. This combination makes it a promising choice for solid-state hydrogen storage solutions in vehicles and portable devices.
Tip: The lightweight nature of lithium borohydride: key role in hydrogen storage solutions helps engineers design systems that are both efficient and easy to transport.
When comparing lithium borohydride: key role in hydrogen storage solutions to other hydrogen storage materials, several advantages become clear. The table below highlights key differences:
| Material | Hydrogen Capacity (wt%) | Operating Temp (K) | Reversibility | Weight Advantage |
|---|---|---|---|---|
| Lithium Borohydride | 18.3 | 289–393 | High | Excellent |
| Sodium Borohydride | 10.8 | 400–500 | Moderate | Good |
| Magnesium Hydride | 7.6 | 573–673 | Moderate | Moderate |
| Metal-Organic Framework | 5–8 | 77–298 | High | Good |
Lithium borohydride: key role in hydrogen storage solutions outperforms many alternatives in both hydrogen capacity and weight. Its ability to store a high level of hydrogen at lower temperatures and its lightweight structure make it a top choice for advanced hydrogen storage solutions.

Researchers have focused on reducing the temperature needed for lithium borohydride to release hydrogen. Lower temperatures make storage systems safer and more energy-efficient. Several methods have proven effective in recent studies. The table below highlights some of the most successful approaches and their results:
| Method/Approach | Effect on Hydrogen Release Temperature | Supporting Data/Results |
|---|---|---|
| TiCl3 doping (2–3 mol%) | Reduced absorption enthalpy | Absorption enthalpy decreased to 40.5 kJ mol−1 at 315–400 °C |
| AlH3 composite | Destabilization, improved kinetics | Released ~11.0 wt% H2 at 450 °C with better kinetics |
| LiBH4-mannitol composite | Low-temperature hydrogen release | Hydrogen release exceeding 7 wt% at low temperature |
| Hydrolysis with H2O (Hδ+ carrier) | Enhanced hydrogen release | Max gravimetric density 7.4 wt% at H2O/LiBH4 = 1.3 mol/mol |
| Transition-metal chlorides doping | Improved hydrolysis kinetics | Max gravimetric hydrogen density up to 8.7 wt% at H2O/LiBH4 = 2–6 mol/mol |
| Alcoholysis with methanol, ethylene glycol | Controllable hydrogen kinetics | Hydrogen release kinetics tunable by alcohol dose and type |
| Nanoconfinement | Lowered hydrogen release temperature | Improved hydrogen storage properties and kinetics |
| Li-Al-Cl compound (plate-like) | Significant reduction in release temperature | Dehydrogenation at 64 °C (2024 study) |
| Fluorographene compositing | Remarkably lowered release temperature | Improved reversible hydrogen desorption performance |
| Metallocene additives | Lower dehydrogenation temperature | Promoted favorable reversibility and kinetics |
Many of these methods, such as nanoconfinement and composite formation, allow lithium borohydride to release hydrogen at much lower temperatures than before. This progress supports safer and more practical hydrogen storage systems.
Efficient regeneration of lithium borohydride remains essential for sustainable hydrogen storage. In 2025, scientists achieved a breakthrough using mechanochemical ball milling. They combined the hydrolytic product LiBO2·2H2O with Al-rich alloys and Li2O additives. This method avoids the high costs and energy demands of older techniques, such as electrolytic reduction or using pure MgH2. The addition of Mg or Ca to Al creates alloys that break apart easily and react well, while Li2O prevents unwanted boron compounds from forming. As a result, this process achieves conversion yields above 40%, making it both cost-effective and efficient. This regeneration strategy marks a major step forward for lithium borohydride recycling and reuse.
Cycling stability measures how well a material performs over many charge and discharge cycles. Recent studies show that modified lithium borohydride systems, especially those doped with borohydride and combined with solid electrolytes like LPSCl, deliver excellent cycling performance. The table below compares the cycling stability of advanced systems to baseline cells:
| Cell/System Type | Current Density (mA cm⁻²) | Cycle Number / Duration | Capacity Retention / Cumulative Capacity | Overpotential / Resistance | Notes / Comparison to Baseline |
|---|---|---|---|---|---|
| 5LBH-LPSCl Symmetric Cell | 2 | 692 cycles / 700 h | 692 mAh cm⁻² cumulative capacity | Lower RSEI+RCT (2.9 Ω) | Stable cycling, no short circuit |
| Baseline-LPSCl Symmetric Cell | 2 | 4 cycles / 13 h | 4 mAh cm⁻² cumulative capacity | Higher RSEI+RCT (7.4 Ω) | Short circuit after 4 cycles |
| Li | 5LBH-LPSCl | Cu Half-Cell | 1 | 50 cycles | N/A |
| Li | Baseline-LPSCl | Cu Half-Cell | 1 | 15 cycles | N/A |
| Anode-Free ASSB (Mg/W-Cu | 5LBH-LPSCl | NMC) | 0.33C (~0.5 mA cm⁻²) | >600 cycles | Capacity retention 79% (125 mAh g⁻¹) |
| High Mass-Loading ASSB | 0.33C | >100 cycles | Capacity retention 92.4% | ICE 75% | Stable cycling at high capacity |
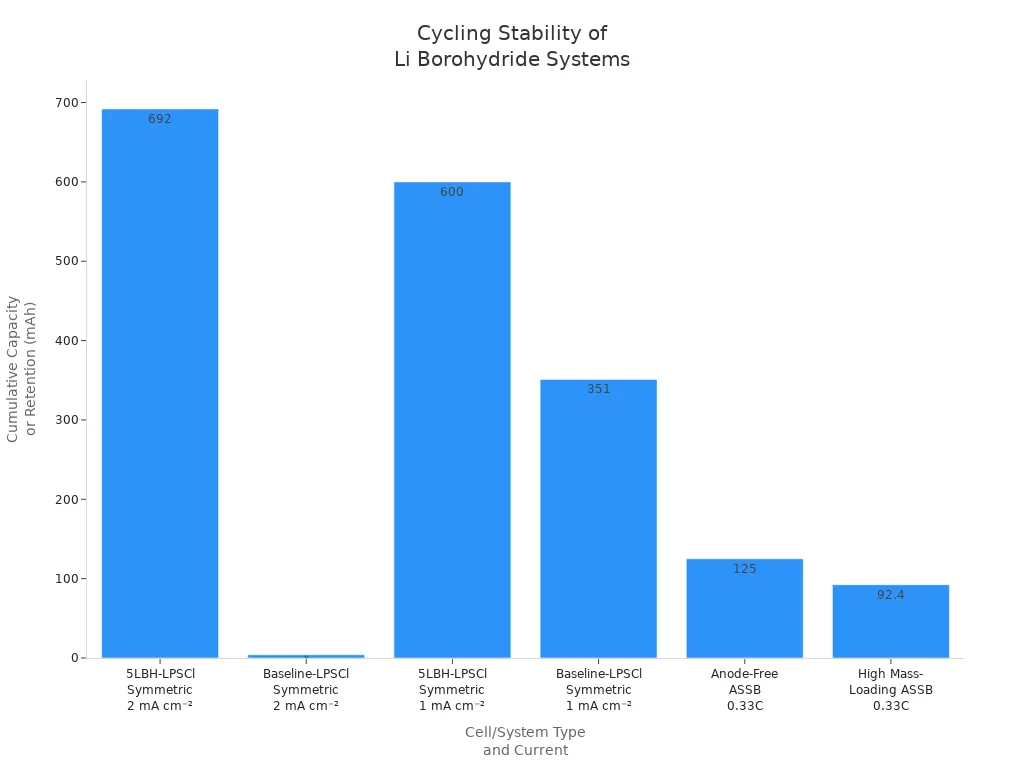
These results show that advanced lithium borohydride systems maintain high capacity and stable operation over hundreds of cycles. Lower resistance and improved efficiency help prevent short circuits and sudden voltage drops. This stability supports the use of lithium borohydride in next-generation solid-state batteries and hydrogen storage devices.
Researchers have made significant progress in improving lithium borohydride hydrogen storage through catalytic enhancement. Catalysts such as carbon materials, metal oxides, and metal borides play a key role in this process. Nickel oxide (NiO) and titanium dioxide (TiO₂) stand out for their ability to lower the activation energy required for hydrogen release. When scientists add these catalysts to lithium borohydride, the temperature needed for hydrogen release drops, and the speed of hydrogen uptake increases. Ball milling lithium borohydride with these catalysts further boosts the hydrogen release rate compared to untreated samples. Nanostructuring and nanoconfinement, when combined with catalytic additives, create even better results. These strategies help overcome slow kinetics and high dehydrogenation temperatures, making lithium borohydride more practical for hydrogen storage.
Tip: Multifunctional catalysts and engineered interfaces can unlock faster hydrogen release and better cycling performance in lithium borohydride systems.
Cation and anion substitution offers another powerful way to enhance lithium borohydride’s hydrogen storage properties. Scientists have studied mixed-metal and anion-substituted borohydrides, such as LiCe(BH₄)₃Cl and LiM(BH₄)₃Cl (where M can be La or Gd). These materials show improved hydrogen storage and higher ionic conductivity. Substituting cations like magnesium, calcium, or zinc, as well as anions such as halides, allows researchers to fine-tune the thermodynamics, kinetics, and stability of borohydrides. This tuning leads to lower hydrogen desorption temperatures, faster reaction rates, and better reversibility. Nuclear magnetic resonance studies reveal that the movement of cations and anions in these salts links directly to improved hydrogen storage and ionic conduction. Research on mixed borohydrides, such as Mg(BH₄)₂–Zn(BH₄)₂, confirms that cation substitution improves stability and hydrogen storage performance. Anion substitution, especially with halides, adjusts stability and enhances dehydrogenation behavior. These modifications help address challenges like high desorption temperatures and slow kinetics, making lithium borohydride more suitable for use in fuel cell vehicles.
Composite formation stands as a leading strategy for boosting lithium borohydride’s hydrogen storage performance. Scientists have achieved major improvements by embedding lithium borohydride into activated carbon, mesoporous carbons, polymer matrices, and nanoporous carbon supports. This nanoconfinement approach enhances hydrogen desorption kinetics, lowers the temperature for hydrogen release, and suppresses the formation of unwanted byproducts like diborane. Metal doping, such as adding nickel, works synergistically to improve reversibility and dehydrogenation. Modification with fluorographite also leads to superior hydrogen release characteristics. First-principles studies show that strong bonding between lithium borohydride and nanoporous carbon supports plays a crucial role in improving thermodynamic properties. These composite strategies address the main challenges of bulk lithium borohydride by improving reaction speed, reducing operating temperatures, and enhancing the ability to reuse the material.
By combining advanced processing and modification strategies, researchers continue to push the boundaries of lithium borohydride hydrogen storage, bringing practical applications closer to reality.
The lithium borohydride market continues to show strong growth as hydrogen storage gains momentum in clean energy sectors. Market analysts project a steady increase in demand, driven by investments in hydrogen storage and battery technology. The following table highlights recent and future market size estimates:
| Period | Market Size (USD million) | CAGR (%) | Notes |
|---|---|---|---|
| 2023 | 58 | N/A | Base year market size |
| 2023–2032 | N/A | 9.2 | Projected CAGR for this period |
| 2032 | 130 | N/A | Projected market size |
| 2024 | 150 | N/A | Base year market size |
| 2026–2033 | N/A | 8.5 | Projected CAGR for this period |
| 2033 | 300 | N/A | Projected market size |
These figures show that the lithium borohydride market will more than double in size over the next decade. The growth comes from rising adoption in hydrogen storage, fuel cells, and battery applications. Companies and governments invest in new technologies to meet the increasing need for efficient and lightweight hydrogen storage.
Cost reduction remains a top priority for the lithium borohydride market. Manufacturers use several strategies to lower expenses and improve competitiveness:
AI and machine learning optimize material design, reducing trial-and-error costs.
Automation in production lines increases speed and consistency, cutting labor costs.
Green chemistry and eco-friendly methods lower environmental compliance and waste management expenses.
Nanostructuring and new composite materials improve stability, making processing more efficient.
Strategic partnerships and investments in research and development focus on better synthesis, stabilization, and recycling.
These efforts help companies produce lithium borohydride more sustainably and at lower cost. The use of renewable energy and recycling also supports sustainability goals and reduces the environmental impact of production.
Several factors drive the adoption of lithium borohydride market solutions worldwide:
The global shift to hydrogen-based energy and renewables increases demand for efficient hydrogen storage.
Fuel cells and hydrogen-powered vehicles rely on lithium borohydride for its lightweight and high-capacity storage.
The electric vehicle market grows, boosting lithium consumption and energy storage needs.
Advances in lithium-based compounds and sustainable chemistry support market development.
New hydrogen production and storage technologies create more opportunities for lithium borohydride market expansion.
Key industries such as aerospace, automotive, electronics, and medical sectors use lithium borohydride for various applications.
Recent research and development in regeneration methods, such as mechano-chemical recycling, make lithium borohydride more viable for commercial use. These innovations reduce costs and close the recycling loop, supporting large-scale adoption. As sustainability becomes more important, companies focus on eco-friendly production and circular economy practices, further strengthening the lithium borohydride market.
Machine learning now drives rapid progress in lithium borohydride hydrogen storage research. Scientists use advanced algorithms to predict hydrogen storage capacities in metal hydrides, including lithium borohydride, by analyzing large datasets. These models identify important material features, such as lattice distortion and thermodynamic properties, that affect hydrogen uptake and stability. Researchers often combine machine learning with density functional theory and molecular dynamics to gain atom-level insights. This approach allows them to screen thousands of material candidates quickly, reducing the need for costly experiments. Deep neural networks and hybrid frameworks handle complex data and improve prediction accuracy. Machine learning also helps design new materials with better hydrogen storage capacity, stability, and faster reaction rates. By using explainable models, scientists can understand how atomic structure influences performance. This innovation shortens the path from discovery to real-world application.
Machine learning reduces the time and cost of developing new hydrogen storage materials, making lithium borohydride more practical for future energy systems.
Lithium borohydride will play a key role in the integration of hydrogen storage with renewable energy sources. As solar and wind power become more common, storing excess energy as hydrogen becomes essential. Lithium borohydride’s high hydrogen capacity and lightweight nature make it ideal for storing and transporting hydrogen produced from renewables. This material supports the shift toward green hydrogen technologies, which use renewable electricity to split water and produce hydrogen without carbon emissions. By enabling efficient storage, lithium borohydride helps balance supply and demand in renewable energy grids. It also supports off-grid applications and remote energy systems. The use of lithium borohydride in these systems advances sustainability and supports the global energy transition.
Lithium borohydride enables flexible storage for solar and wind energy.
It supports the use of hydrogen as a clean fuel in transportation and industry.
The material’s recyclability aligns with circular economy goals.
Safety remains a top priority as lithium borohydride moves closer to widespread use. Researchers focus on improving the stability of lithium borohydride during storage and handling. They develop new composite materials and protective coatings that reduce the risk of unwanted reactions. Advances in nanoconfinement help control the release of hydrogen, preventing sudden pressure changes. Scientists also use sensors and smart monitoring systems to detect leaks or temperature spikes early. These improvements make lithium borohydride safer for use in vehicles, portable devices, and large-scale storage facilities. Ongoing research aims to meet strict safety standards while maintaining high performance.
Continued progress in safety will build trust and support broader adoption of lithium borohydride in hydrogen storage applications.
Researchers in 2025 have transformed lithium borohydride into a leading hydrogen storage material.
Composite modifications, nanoconfinement, and digital alloy design have improved efficiency and safety.
The market for lithium borohydride continues to expand as new applications emerge.
Future research will likely unlock even greater performance.
Hydrogen storage innovation remains essential for a clean energy future. Scientists and engineers should stay engaged with these exciting developments.
Lithium borohydride stores hydrogen in a solid form. Engineers use it in fuel cells, batteries, and portable energy devices. Its high hydrogen content and lightweight structure make it ideal for advanced energy systems.
Nanoconfinement traps lithium borohydride in tiny pores. This process lowers the hydrogen release temperature and speeds up reaction rates. The material cycles more efficiently and resists clumping.
Researchers design new composites and coatings to improve safety. Proper storage and handling reduce risks. Safety sensors and monitoring systems help detect leaks or temperature changes early.
| Material | Hydrogen Capacity (wt%) | Operating Temp (K) |
|---|---|---|
| Lithium Borohydride | 18.3 | 289–393 |
| Sodium Borohydride | 10.8 | 400–500 |
| Magnesium Hydride | 7.6 | 573–673 |
Lithium borohydride offers higher capacity and operates at lower temperatures.
Yes. New regeneration methods, such as mechanochemical ball milling, allow efficient recycling. These processes reduce costs and support sustainable hydrogen storage.
Aerospace, automotive, electronics, and renewable energy sectors benefit most. These industries need lightweight, high-capacity hydrogen storage for advanced applications.